Immunogenicity
Immunogenicity

Discovery Sciences
Protein Sciences
Antibody Libraries
In Vitro Immunology
Immunogenicity
Immuno-functionality
Our Approach
ImmunAcademy
Additional Specialty Testing Services
ImmunXperts’ immunogenicity testing allows you to de-risk your pipeline and optimize candidates for reduced adverse events and lower immunogenicity risk. Choose from a comprehensive immunogenicity assessment or just the components you need.
In silico methods for low-cost, high-throughput assessment
- Computer-based assessment of T-cell epitopes for HLA-binding risk
- Sequence-based assays enable rapid assessment of large numbers of lead candidates
- Useful for deimmunization (lowering unwanted immunogenicity)
In vitro early/innate assays
Our in vitro assays include flow cytometry, cytokine profiles, and ELISpot options
- Cytokine release assay – using whole blood or PBMCs (human and NHP); customizable up to 60 cytokines
- Monocyte activation assay
- Dendritic cell (DC) activation/maturation assay
- Reporter cell line assay
In vitro T-cell assays
- CD8-depleted PMBC assay – T-cell proliferation by flow cytometry
- DC/T-cell assay – appropriate for immunomodulatory molecules known to directly interact with T cells
- Peptide assay – ultrasensitive Fluorospot assay; customizable up to 4 cytokines
MHC-associated peptide proteomics (MAPPS Assay)
- Highly specialized technique using mass spectrometry to determine which peptides are presented on the cell surface on MHC molecules; often followed by a peptide assay to evaluate T-cell response and identify the immunodominant epitopes
Anti-drug antibody (ADA) assay
- Evaluates pre-existing anti-drug antibodies present in global or specific populations
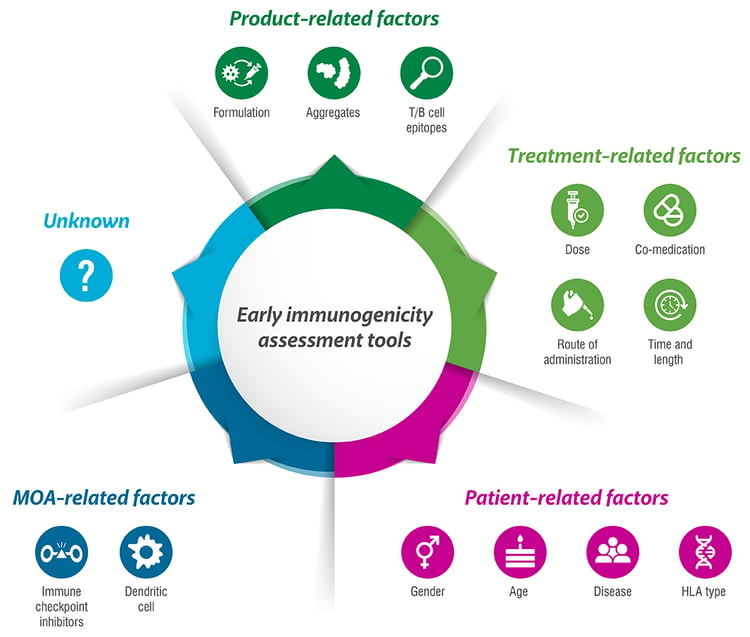
The Value of Immunogenicity Assessments
The key process in T-cell dependent antibody production is peptide presentation by antigen presenting cells to T cells, in combination with co-stimulatory signals and cytokine secretion. These three signals stimulate the T cells to proliferate and differentiate, helping the B cells produce antibodies toward the antigen.
Antibodies produced toward an administered drug (anti-drug antibodies or ADAs) are the main clinical immunogenicity problem. These ADAs can alter drug pharmacokinetics and pharmacodynamics, and negatively affect safety and efficacy. If the drug mimics an endogenous counterpart, the ADAs might also bind the endogenous protein, which can lead to severe adverse events. The process from antigen presentation until ADA production cannot (yet) be tested in vitro. Therefore, T cell proliferation is used as a surrogate marker to measure the immunogenic potential of a drug candidate in development. T-cell activation assays have a long tradition in vaccine testing (wanted immunogenicity) and can also be employed to assess possible unwanted immunogenicity induced by therapeutic proteins.